Treat
People
Better.
It’s Not Rocket Science
Treating people better starts with rethinking how medicines are delivered. Current Intranasal device innovation has not kept pace with therapeutic needs.Medicines work best when dosing is precise and consistent, side effects are minimal, treatment targets the intended site, and therapy is accessible and easy to use.
The delivery method of the medicine plays a fundamental role in all of these success factors. Enter the nasal cavity. The nasal cavity is a promising route for drug delivery for a wide range of treatments, with different subregions of the nasal cavity providing efficient pathways to the brain, the immune system, and the bloodstream. To date, complex nasal anatomy has been a major barrier to successfully exploiting the nasal cavity for medicine delivery.
Rocket Science Health has broken through. Our drug delivery device technology precisely targets the nasal subregions ensuring medicines reach their intended destination. Designed around patient need, the device is intended for at-home use, even for those with cognitive and physical impairments.
TREAT
Precise and consistent dosing to the right target
PEOPLE
Seamless fit and easy to use
BETTER
Empowering patients, improving access and clinical outcomes
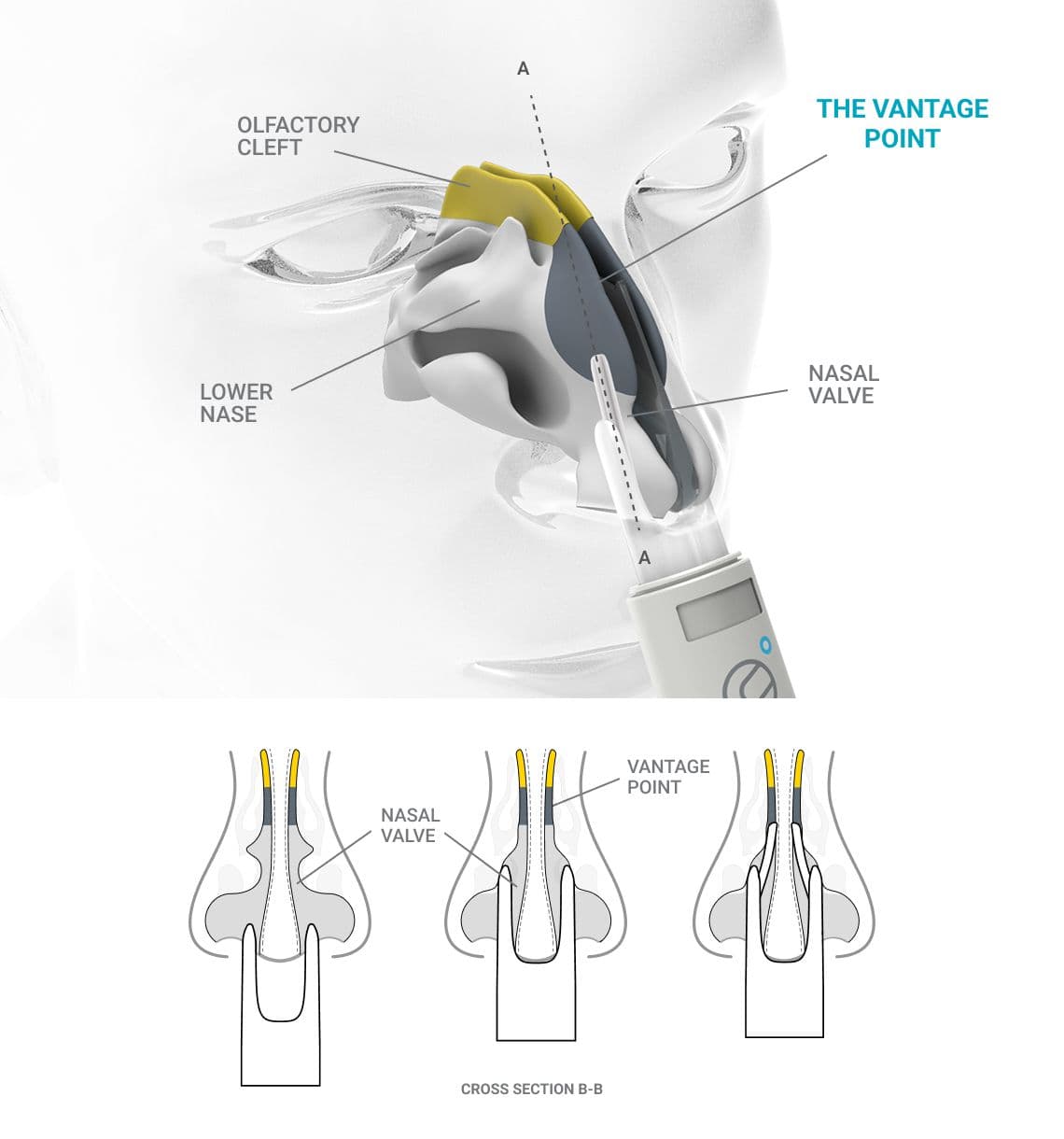
Rocket Science Health VANTAGE POINT
TREAT PEOPLE BETTER
Treatment starts with precise and consistent dosing to the target
The answer is Rocket Science Health patented technology:
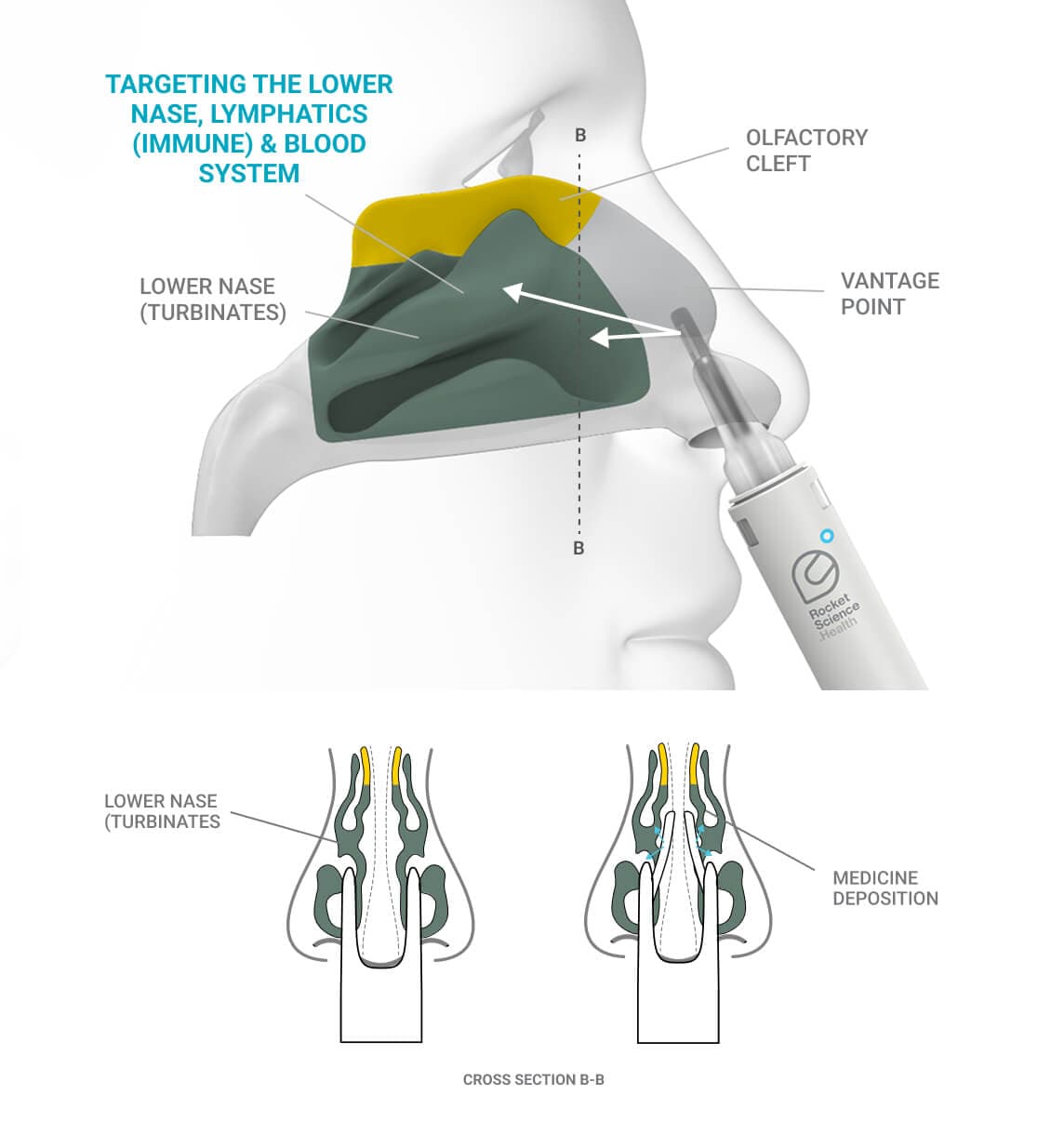
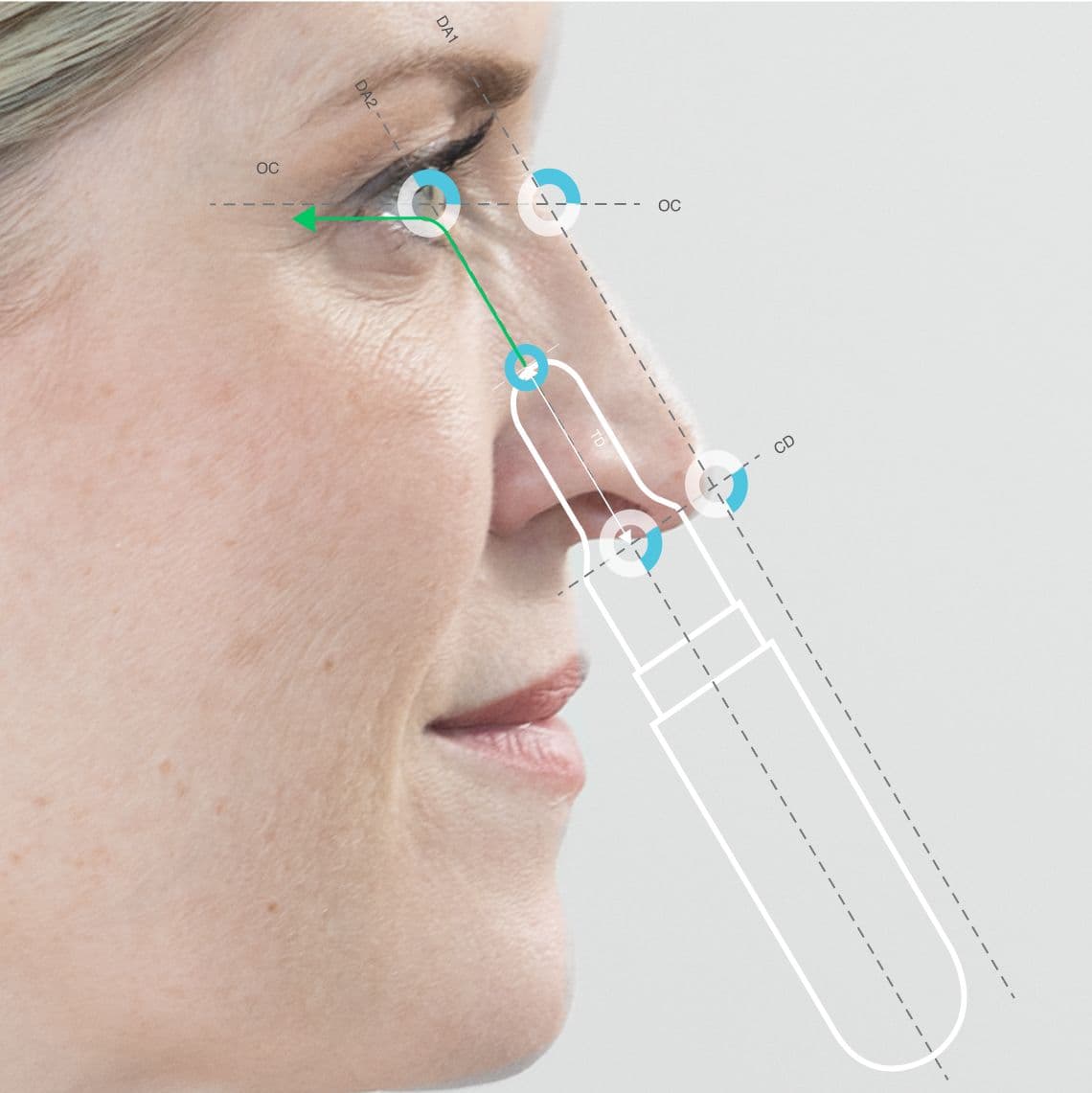
Vantage point - the device architecture includes nasal applicators that reach above the nasal valve with a clear line of site to the target.
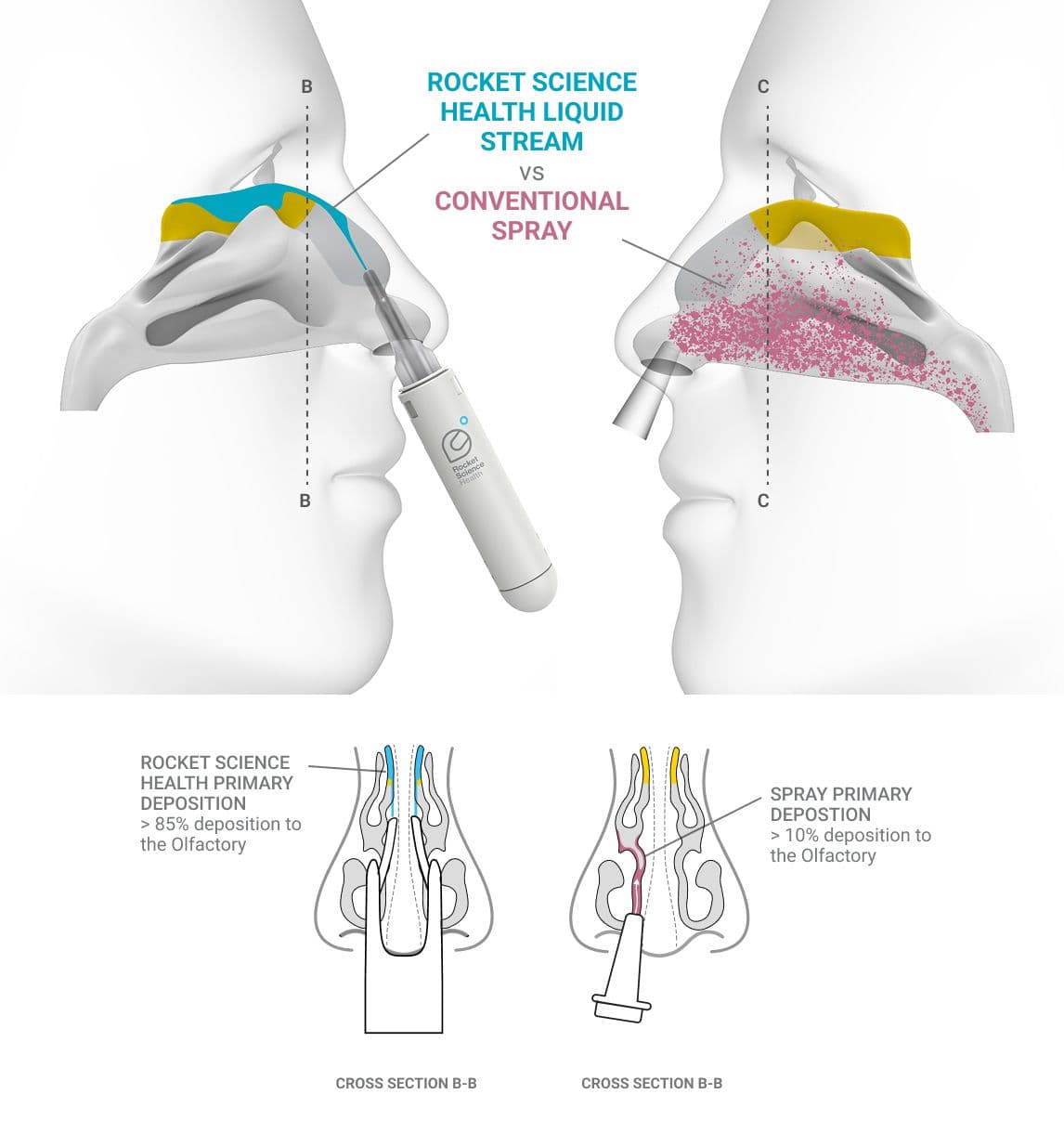
Fluid dynamics - the device delivers a cohesive fluid stream—rather than a traditional spray—once correctly positioned. This targeted stream directs the medication precisely to the intended area, minimizing off-target waste. Additionally, unlike conventional nasal sprays, the fluid stream operates with low mechanical force, preserving the integrity of fragile drug molecules such as mAbs and biologics.
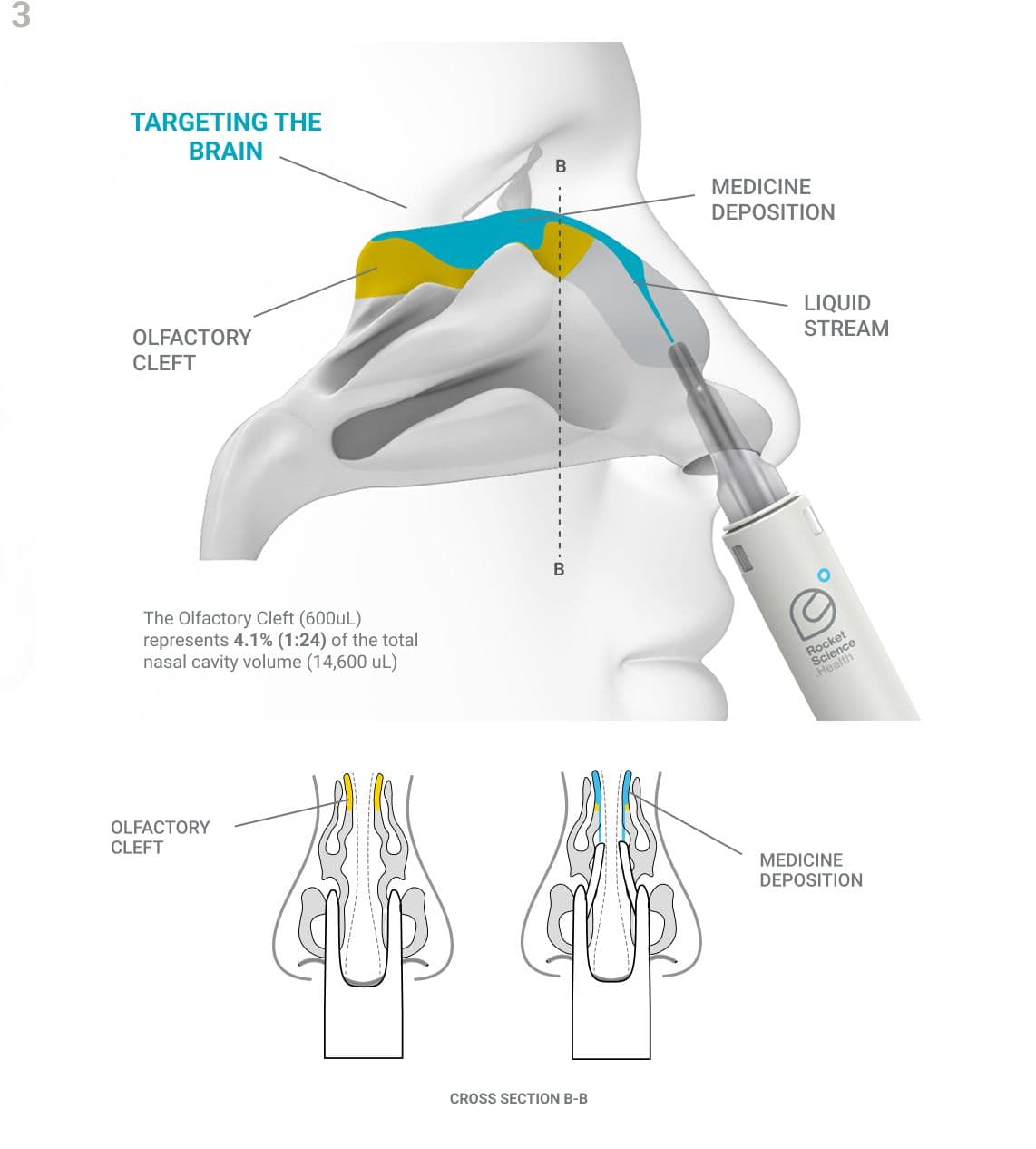
The Olfactory Cleft - a small but crucial part of the nasal anatomy. Although difficult to access and holding only 1:24 the volume of the rest of the nasal cavity, it serves as the most efficient—and in many cases the only—non-invasive pathway to the brain for certain medicines.
LEARN MORE:
HFUE FORMATIVE STUDIES
TREAT PEOPLE BETTER
Our design, driven by patient needs
Human-centered design was key to Rocket Science Health’s technology R&D process. From day 1, we have sought to ensure that all people can access, experience and benefit from these advanced treatments.
Key design elements:
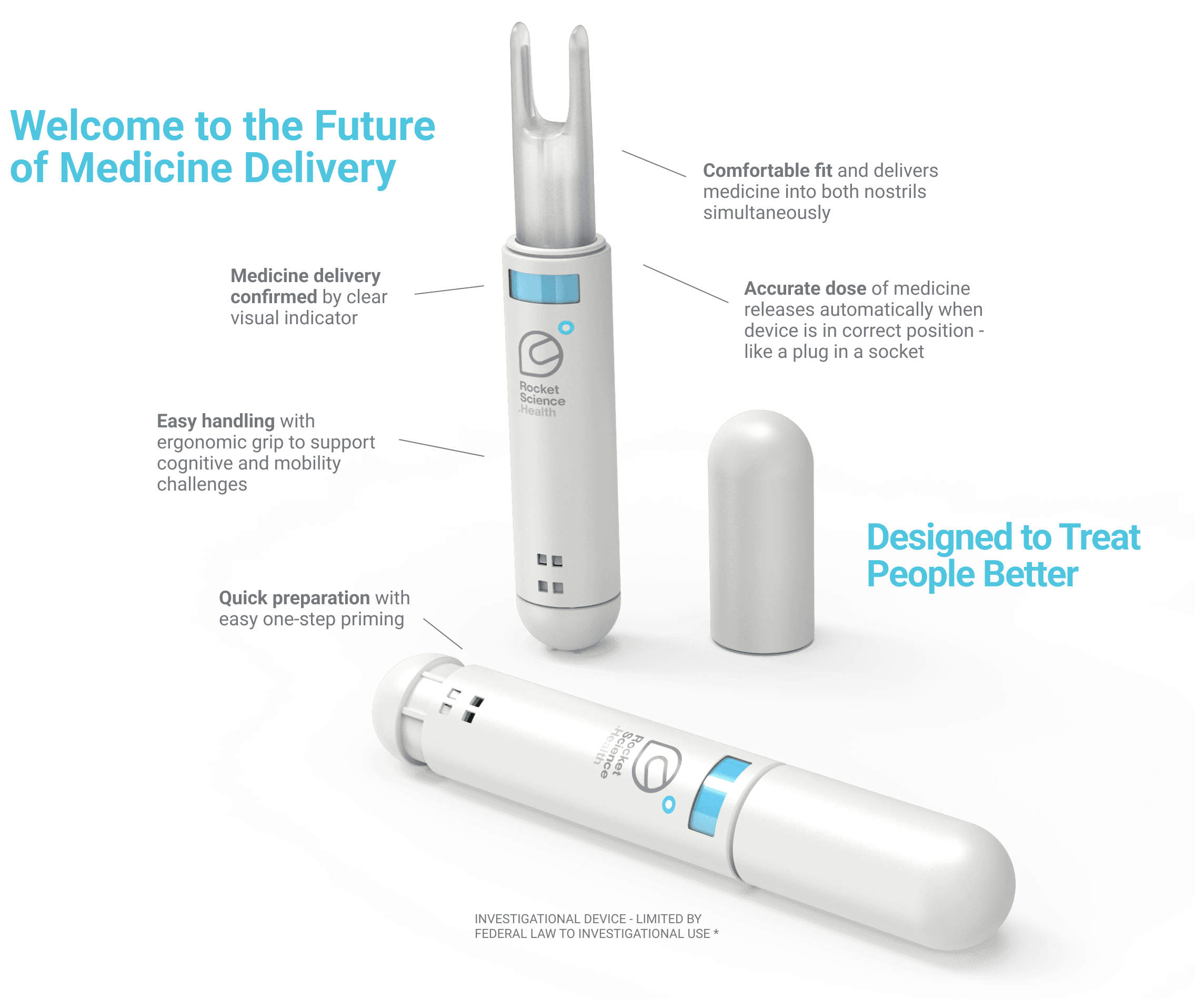
Works across diverse nasal anatomies based on age, race, and gender.
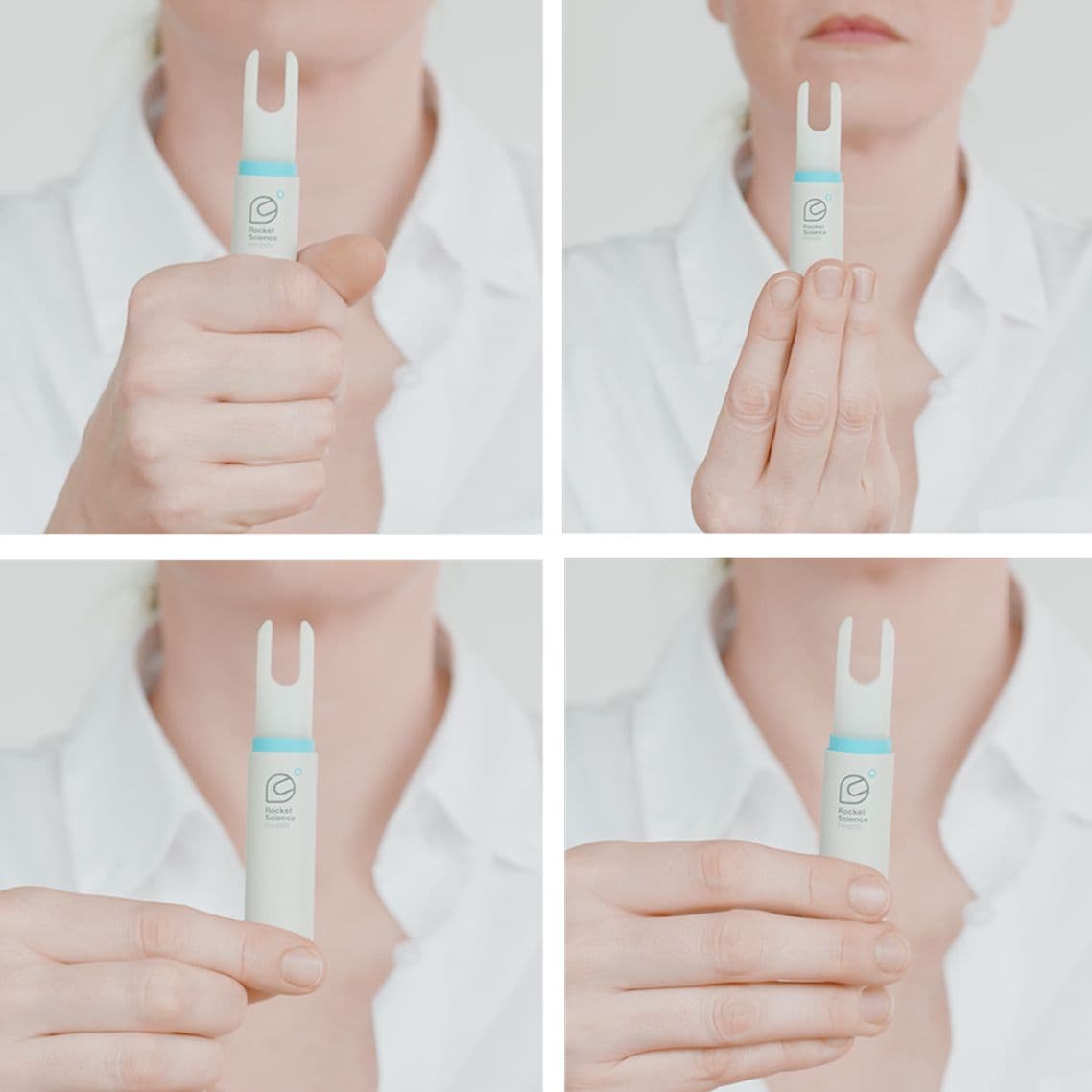
Intuitive, easy to pick-up and naturally orient, especially for patients with cognitive or physical impairment.
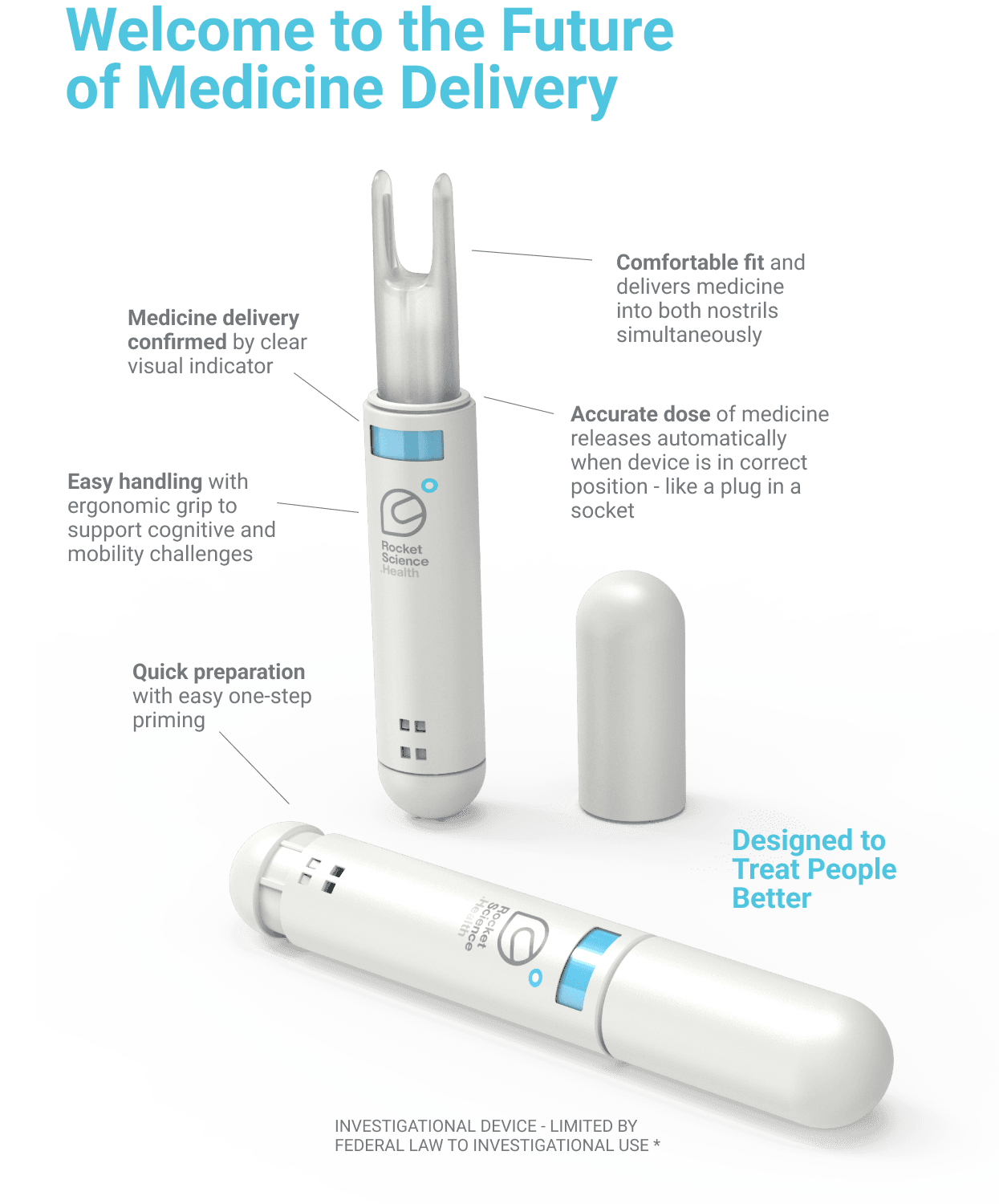
Comfortable fit, depth and angle of insertion for accurate repeatable dose ‘like a plug in a socket’.
Eliminates force dependency - automatically delivers the correct amount of medicine once in place, without the need for awkward squeezing or pump action.
SELF-GUIDED INSERTION & ADMINISTRATION
TREAT PEOPLE BETTER
Better clinical outcomes across a range of treatments
This is our ambition as clinical data is pending. Our intent is to make life changing treatments more approachable, easy to use, and most importantly, capable of delivering better clinical outcomes. Supporting a new standard of care across many treatment areas, Rocket Science Health’s device technology is adjustable across dose volume, viscosity, and single or multi-dose usage.
Here’s whats better:
Agency: self-administration with clinical precision, patients gain control of their environment not having to rely on visits to the clinic, saving time, money, and stress.
Access: a non-invasive, needle-less treatment option has the potential to replace needle-administration and invasive surgery.
Adherence: an ‘always-with-you' device improves treatment adherence. The Rocket Science Health product line includes a proof-positive software app that verifies patient and proper, timely treatment that may be shared with the care team, and caregivers.
Application: Extensive R&D, both 1st and 3rd party, point to this technology and route of administration improving clinical outcomes and enabling a range of treatment areas.
Investigational Device - Limited by Federal Law to Investigational Use *
PARTNERSHIP OPPORTUNITY
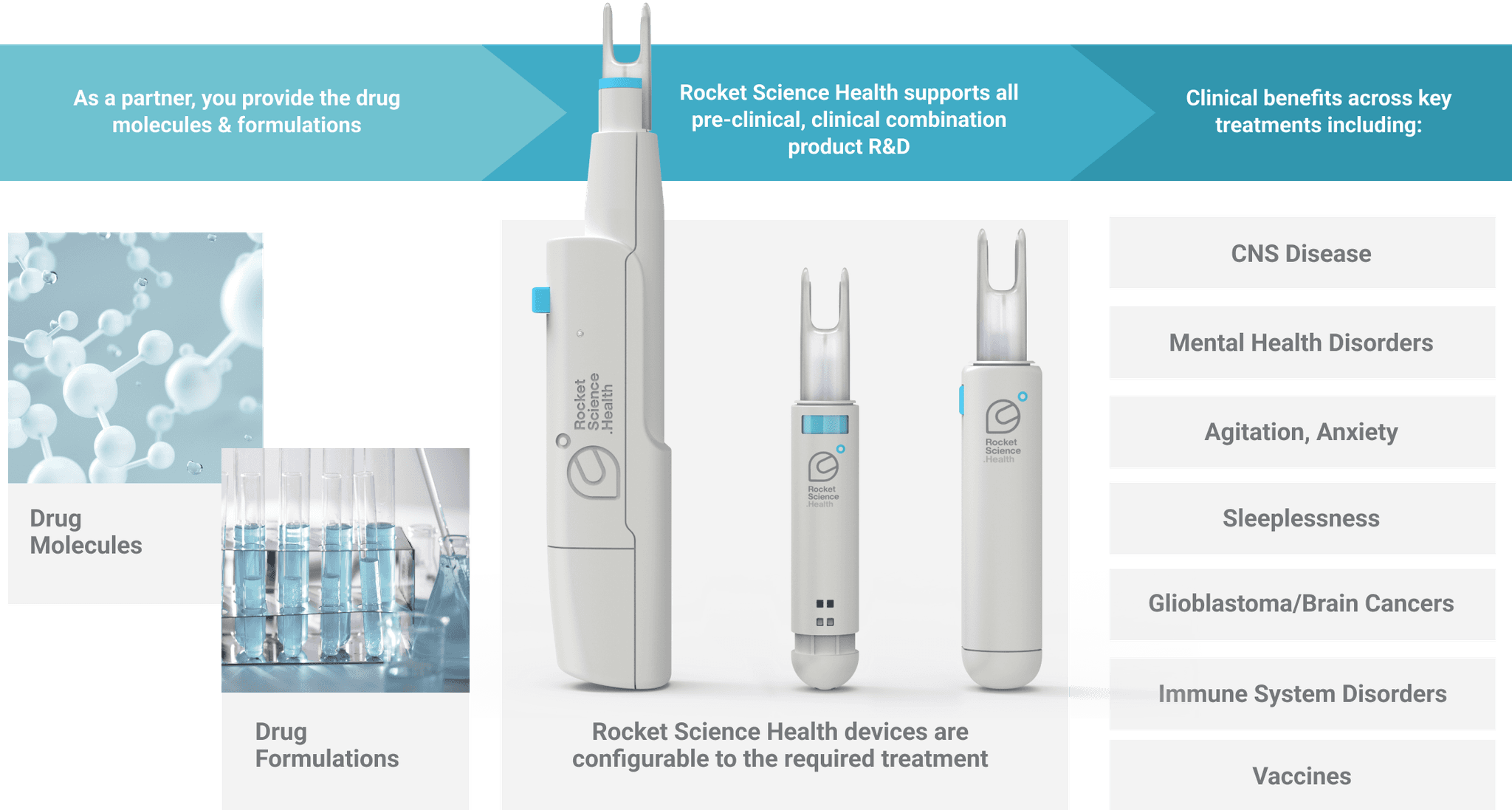
Partner with us to Treat People Better
For our partners, the route of administration and device are key to your medicine’s clinical benefit and commercial successWe engage with partners early and often on combination product development programs. Our device technology plays a central role in validating and optimizing drug molecule and formulation efficacy at every stage of the R&D process.
PARTNERSHIP OPPORTUNITY
Accelerate your pipeline and grow commercial value
We help our partners create, lead and extend combination product commercialization across existing and new drug moleculesAs your device-centered combination product partner, Rocket Science Health will work with your preferred R&D and CDMO partners for product commercialization. Or, we will call on our network of experts from across the supply chain in order to meet your requirements. Our deep IP estate and rigorous human factors orientation enables you to differentiate and lead in a competitive market. Whether it’s a new molecule or a 505(b)(2) pathway, we adapt to your pipeline.

ROCKET SCIENCE HEALTH
About Us
Rocket Science Health develops precision drug delivery technologies focused on brain-targeted therapeutics and vaccines. Our needle-free intranasal device enables non-clinical self-administration for at-home care, emergency rescue, and mass immunization. Its fluidics are specifically engineered for today's delicate biologics and dose-dependent drugs.
Leadership:
Kenneth Irving, Founder
Sohier Hall, President

Ready to Engage?
Send us a signal.
Contact us at hello@rocketscience.health to explore opportunities. We’re ready to share our learnings and discuss your needs, including:
• Learning about our advancements in intranasal drug delivery
• Expanding your IP portfolio
• Initiating a clinical trial
• Engaging in human factors testing
• Sharing data and clinical trial evidence
Your information will be kept confidential and used solely for staying in touch. We will not share your details with third parties. For more information, please see our Privacy Policy.